Abstract
Background: Studies on TP53 mutations as prognostic markers in diffuse large B-cell lymphoma (DLBCL) have historically been controversial and these results have not been consistent across different studies. Considering the complex pathophysiological mechanisms involved in DLBCL, we assumed that the interaction of TP53 with other genetic variants, which shape the discrepant immune landscape, could further promote the development of DLBCL, and thus be more prognostically predictive.
Materials and methods: Comprehensive analysis of the genomic characteristics of TP53 and CD58 was performed through high-throughput DNA (n=176) and relevant RNA (n=152) sequencing in patients with de novo DLBCL. Mutation data of 1,001 DLBCL patients from Duke University's cohort were downloaded for validation. Another validation cohort was from REMoDL-B trail (N=400).
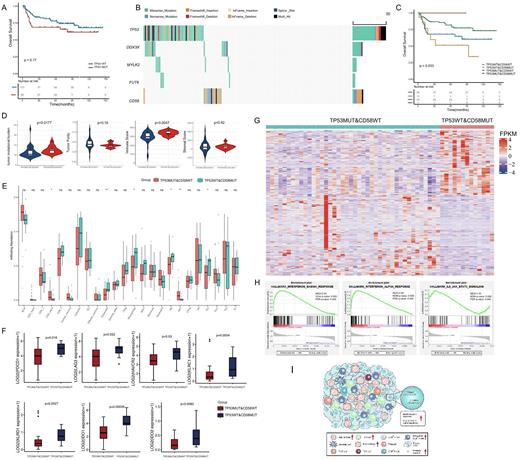
Results: A total of 227 significantly mutated genes were identified, of which TP53 was the second most frequently mutated gene, with a rate of 30% (53 of 176) and 62 sequence variants detected. Among these variants, 74% were missense mutations, and the remaining were inactivating frameshift indels, nonsense mutations, coding sequencing indels and splicing mutations. Importantly, most mutations (87.5%) occurred in exons 5-8, which encoded the DBD region of TP53. Codons 175, 273, and 248 of the p53 protein had the highest mutation frequency. However, TP53 mutations alone are insufficient to effectively differentiate the risk of DLBCL, even when only considering mutations in the DBD region. We further found that DDX3X, MYLK2, and FUT6 mutations co-occurred with TP53 mutations, and CD58 mutations were mutually exclusive with TP53 mutations. No significant difference was observed in survival among those groups according to the combination of co-occurring mutation genes with TP53 (p=0.37 for DDX3X; p=0.11 for MYLK2; p=0.54 for FUT6). However, the combination of TP53 and CD58 mutations could significantly distinguish the prognosis of patients with DLBCL (p=0.033). Patients with both wild-type TP53 and CD58 had a better prognosis than patients with either of the two mutually exclusive modes of CD58 and TP53 mutations. Unexpectedly, patients with TP53 wild-type and CD58 mutations (TP53WT&CD58MUT) had worse survival than those with TP53 mutations and CD58 wild-type (TP53MUT&CD58WT). The relationship of a mutually exclusive mutant between CD58 and TP53 and the prognostic significance of this interaction was validated using publicly available data from 1,001 patients with DLBCL from the Duke university's cohort.
We then explored whether the cooperation of the mutually exclusive mutations between TP53 and CD58 may profoundly influence the microenvironment in DLBCL. We found that the overall TMB was significantly higher in the TP53WT&CD58MUT group than in the TP53MUT&CD58WT group (p=0.0177), while there was no difference in TMB when dividing patients only according to TP53 mutation status (p=0.5348). In addition, the ESTIMATE immune scores in the TP53WT&CD58MUT group were significantly higher than that in the TP53MUT&CD58WT groups (p=0.0047). Moreover, the exhausted T cell, macrophage cell, NK cell, and Th1 cell enriched in the TP53WT&CD58MUT group. The difference between the two groups was mainly due to the combined influence of the mutation pattern 'TP53WT&CD58MUT', but rather only affected by the CD58 mutations, given the immune cell infiltration was similar when dividing patients just according to CD58 mutation status. Furthermore, the co-inhibitory receptors such as PD-1, TIM3, and LAG3 were preferentially expressed in the TP53WT&CD58MUT group. Inhibitory immunomodulators were also significantly upregulated in the TP53WT&CD58MUT group when comparing with the TP53WT&CD58WT group, suggesting the unique immune phenotype in the TP53WT&CD58MUT group. The findings that high immune scores and abundant infiltrating exhausted T cells in the TP53WT&CD58MUT group were validated in an independent external cohort from the REMoDL-B trail (N=400). GSEA showed significantly activated interferon-α and -γ responses and IL-6/JAK/STAT3 signaling in the TP53WT&CD58MUT group.
Conclusions: Our findings suggest that TP53 mutation alone is insufficient to effectively differentiate the risk of DLBCL. The mutually exclusive patterns between TP53 and CD58 mutations accurately stratified patients with DLBCL to permit the optional immunotherapy.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal